By Barry Keate
Barry Keate, has lived with tinnitus over 40 years and has published 150+ research articles on numerous aspects of tinnitus. He is an expert on the condition and a well-known advocate for those with tinnitus.
A recent clinical study was conducted on the efficacy of an experimental tinnitus medication called AM-101. The study was the first large-scale study using this medication on humans and established proof of concept for AM-101 in the treatment of tinnitus arising from glutamate excitotoxicity. (1)
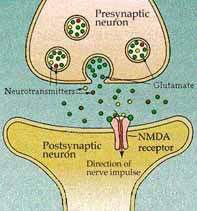
AM-101 (from Auris Medical AG, Basel, Switzerland) is an NMDA receptor antagonist that is being developed for treatment of acute inner ear tinnitus. The NMDA (N-methyl-D-aspartate) receptor is a glutamate receptor on the neuron leading to the auditory cortex that is communicated to by the hair cells of the inner ear.
By being an NMDA receptor antagonist, the concept is that AM-101 will block excess glutamate from binding to the neuron, thereby preventing glutamate excitotoxicity and the neuronal death that follows. This should allow for regrowth and synaptic repair of auditory neurons during the early stages of acute tinnitus. AM-101 aims to treat tinnitus in the acute stage before it becomes centralized or memorized at higher structures of the auditory system or the brain.
This last point is very important: AM-101 is designed as an early intervention in cases of early onset noise-induced hearing loss and tinnitus. It will not be an effective tinnitus therapy for chronic, long standing tinnitus. All the participants of this study had tinnitus for less than three months.
Glutamate Excitotoxicity
We have discussed glutamate excitotoxicity in depth in previous articles. The discussion also included a paragraph on an earlier study of AM-101 on laboratory animals. Essentially, glutamate is an excitatory neurotransmitter that is necessary to carry electrical impulses from the hair cells in the inner ear to the neurons leading to the auditory cortex in the brain.
When the hair cells are damaged due to exposure to loud noise, ototoxic medications or other causes, they emit an excess of glutamate, sometimes called a glutamate storm. This excess glutamate overexcites the neuron causing it to fire electrical pulses until it becomes chemically depleted, at which point it dies.
When the neuron dies it ceases to transmit sound impulses to the neurons in the auditory cortex. These neurons are organized according to sound frequency, meaning each neuron responds to a specific frequency of sound. When there is no incoming signal at that frequency the receiving neuron might well respond to a signal in the surrounding region even though it is a different frequency. However, since the neuron can only relay one frequency it will create a continuous sound in the frequency for which it is attuned even though there is no incoming signal from that frequency. This is what we know as tinnitus.
Tinnitus Treatment with AM-101
The clinical study referred to enrolled 248 patients with three types of tinnitus; acute acoustic trauma (noise exposure), sudden sensorineural hearing loss (SSHL), and tinnitus caused by acute otitis media (infection of the middle ear). Although not frequently discussed, it has been postulated that infection in the middle ear can produce an inflammatory molecule called a cytokine, which migrates to the inner ear and can trigger glutamate excitotoxicity.
The patients were treated with AM-101 on the beginning day, again on day 1 after and again on day 2 after. The drug was administered by transtympanic injection (through the ear drum) under local anesthesia. The patients would tilt their heads 45 degrees toward the unaffected ear and remain in that position for 30 minutes to allow the substance to diffuse into the cochlea.
The patients visited the clinic on five occasions; for the initial three injections and on days 30 and 90 after the injections. On each visit they were submitted to a series of tests to measure loudness match, tinnitus annoyance, sleep difficulties, the Tinnitus Handicap Inventory (THI) and Minimum Masking Levels (MML) . MML is a standard audiometric test that determines the lowest level at which a standard band of noise renders tinnitus inaudible. The primary end point of the study was considered to be changes in Minimum Masking Levels.
The study overall failed to demonstrate a treatment benefit based on change in Minimum Masking Levels. However, AM-101 showed statistically significant improvement for tinnitus loudness, annoyance, and sleep difficulties on patients with noise exposure and infection in the middle ear. The group of patients with sudden hearing loss had inconclusive results. The researchers speculated this might have been due to a high percentage of spontaneous remission in this group.
Improvement in tinnitus loudness of at least 50% was seen in 42% of the medicated group compared to 14% of patients in the placebo group. Approximately 57% of patients in the medicated group rated their tinnitus severity at day 90 compared with baseline as “much improved” or “very much improved.”
Improvement in tinnitus was gradual over the 90-day observation period. In the first days tinnitus loudness increased slightly as the eardrum was still open and sound perception may have been temporarily altered.
There are several unanswered questions that arise from this study. First, for how long will the injections prevent tinnitus from returning? It is speculated that the AM-101 stops tinnitus from becoming memorized in the auditory cortex and allows for regrowth and synaptic repair of auditory neurons but this has not been proven. Second, for how long after glutamate excitotoxicity begins will the medication be helpful? In this study, the average time with tinnitus was 61 days. Third, what is the most effective dosage? Here the higher of two dosages was 0.81 mg/ml but its possible a higher dosage would be more effective. Larger and longer-term studies are needed to answer these questions.
An Alternative Tinnitus Treatment
As we have discussed previously, the Ginkgo biloba found in Arches Tinnitus Formula is a powerful glutamate antagonist and helps to reduce excessive glutamate and prevent excitotoxicity. For several years Arches has been using an advanced form of Ginkgo extract, which has higher concentrations of the ginkgo flavone glycosides and terpene lactones than normal. We have received trademark approval from the US Patent and Trademark Office for Ginkgo Max 26/7® to describe our product.
Ginkgo Max 26/7® is a higher concentrate of Ginkgo Biloba Extract than the common form designated as 24/6. This indicates the ginkgo flavone glycosides comprise 24% of the common form but a minimum of 26% in Ginkgo Max 26/7. Likewise, the terpene lactones comprise 6% of the common form but a minimum of 7% in Arches ginkgo.
Perhaps more importantly for this discussion is the concentration of bilobalide in the ginkgo. Bilobalide is the fraction of Ginkgo biloba found in the terpene lactones that is responsible for neuroprotection and glutamate antagonism. The common form of ginkgo is specified to contain a minimum of 2.6% bilobalide, however most of the retail products sold in the US have between 0.5% and 2%. The ginkgo in Arches Tinnitus Formula contains a minimum of 3.5% bilobalide providing a high level of neuroprotection and glutamate antagonism.
Please view a recent Certificate of Analysis for Arches Ginkgo biloba extract.
Two advantages Arches Tinnitus Formula has over AM-101 is it does not have to be used within a short period of time after the damage occurs, nor does it require injections to the eardrum. Although it has been noted that better outcomes occur when used within the first year, many people find relief after a long period of continued tinnitus. I had severe tinnitus for 20 years before using this product and have still had a remarkable reduction in the sound level. Darius Kohan, MD, a noted Ear, Nose and Throat physician, has reported that in those with noise-induced hearing loss and tinnitus, he has a success rate of 75-80% in significantly reducing tinnitus loudness using Arches Tinnitus Formula.
Obtaining these higher extract levels of the active ingredients is expensive as more raw material is required. But it is absolutely essential for those of us who have intractable tinnitus. This is why people who use Arches Tinnitus Formula and change to off-the-shelf brands inevitably return to us. Superior ingredients produce superior results.
References:
1 – van de Heyning P, Muehlmeier G, Cox T, Lisowska G, Maier H, Morawski K, Meyer T. Efficacy and Safety of AM-101 in the Treatment of Acute Inner Ear Tinnitus – A Double-Blind, Randomized, Placebo-Controlled Phase II Study. Otol Neurotol 35:589-597, 2014.
Get Free Shipping!
Order now and get free shipping on either the Tinnitus Starter Kit or Combo Pack. Try the doctor recommended products with clinically proven ingredients for tinnitus. No coupon code required.